The Expanding Universal Drug Table
CONTEXTA foundation for ultimate interoperability
SmithRx envisioned the Universal Drug Table (UDT) as the foundational system that would power operations across the entire business. With hundreds of thousands of drugs utilized across multiple products, UDT would be the single source of truth that every department and system would depend on. When members checked drug prices, when claims were processed, when Connect programs saved patients thousands—everything would trace back to UDT.
CHALLENGEGhosted by its own team
After months of development, UDT adoption remained nonexistent. Leadership feared they’d severely misfired. Critical initiatives were stalling, and tensions with Patient Access were heated.
Under immense pressure, executives demanded to know what was delaying the Patient Access and Operations teams’ migration. They responded: “It’s broken.”
The firestorm of a conversation that followed must have been harrowing—because that’s when I received a direct Slack message outside the standard process.
Leadership wanted someone to drop everything and dive in. Immediately.
DISCOVERYThe UI without a user
There was no handoff. No backlog. Just a broken tool and rising tension across multiple teams. So I went to the source—sat in the friction, mapped the workflows, and listened as Patient Access and Operations flooded me with context. A clearer picture began to emerge: what UDT was meant to solve, and where it was failing in its current form.
Ops and PAS utilized standard terminology, sourced from a wide array of suppliers, and update records at a categorical level, rarely a singular NDC.
What I uncovered was staggering: a system built for no one. One-at-a-time editing that would have multiplied Operations’ workload. Cryptic database fields with no context. No governance. No validation. A single transposed decimal could be devastating.
Teams maintained over 250,000 drug records across 30+ Google Sheets
It was a systemic failure—neither team could complete a core function in UDT. They had resorted to managing 250,000+ drug records across 30+ Google Sheets to keep the business running.
Ops managed the data and then repeated tasks across SmithRx products. UDT was intended to centralize data as a single source of truth, but failed.
Ops managed the data and then repeated tasks across SmithRx products. UDT was intended to centralize data as a single source of truth, but failed.
UDT—built to replace spreadsheets—ended up spawning new ones.
UDT exposed raw fields like gpi6_txt and cpm_t_yn, leaving Ops and PAS to reverse-engineer their own definitions in yet another Google Sheet. Single-drug lookup? Useless for batch work. The system didn’t just miss — it made their jobs harder.
The interface had capability—but no clarity. It was functional, but functionally meaningless.
The chaos wasn’t their fault — it was the system’s. But buried inside it was signal. I surfaced four persistent themes to shape the path forward.
-
250,000 active drugs × 108 attributes = 27 million data points at risk
-
Manual decimal entry, no validation.
$20.39 → $203.70 near-misses documented.
-
Bulk operations dominated—assignments, status updates, pricing changes.
-
1–2 hours spent on remedial tasks that could’ve taken minutes.

From database structures to human-centricity
Leading the entire discovery and design process from scratch, I rebuilt UDT's foundation on four principles that would enable complete transition from third-party spreadsheet workflows. This wasn't about features—it was about translating system logic into human thinking so Ops and PAS teams could rely on UDT for their entire drug data workflow.
-
Replace database codes with language ops teams actually use (NDCs, GPIs, status names)
-
Design for hundreds of simultaneous updates, not single records
-
Context-aware error prevention (10% price change warnings, decimal validations)
-
Pre-configured views and filters for common task patterns
The objective: create the definitive drug data tool by mapping operational needs into natural, efficient processes.
STRATEGY
Introducing Precision Drug Management
Precision Drug Management (PDM) transformed UDT from data repository to workflow intelligence system. Every feature anticipated real work patterns, prevented real errors, and made the right actions inevitable.
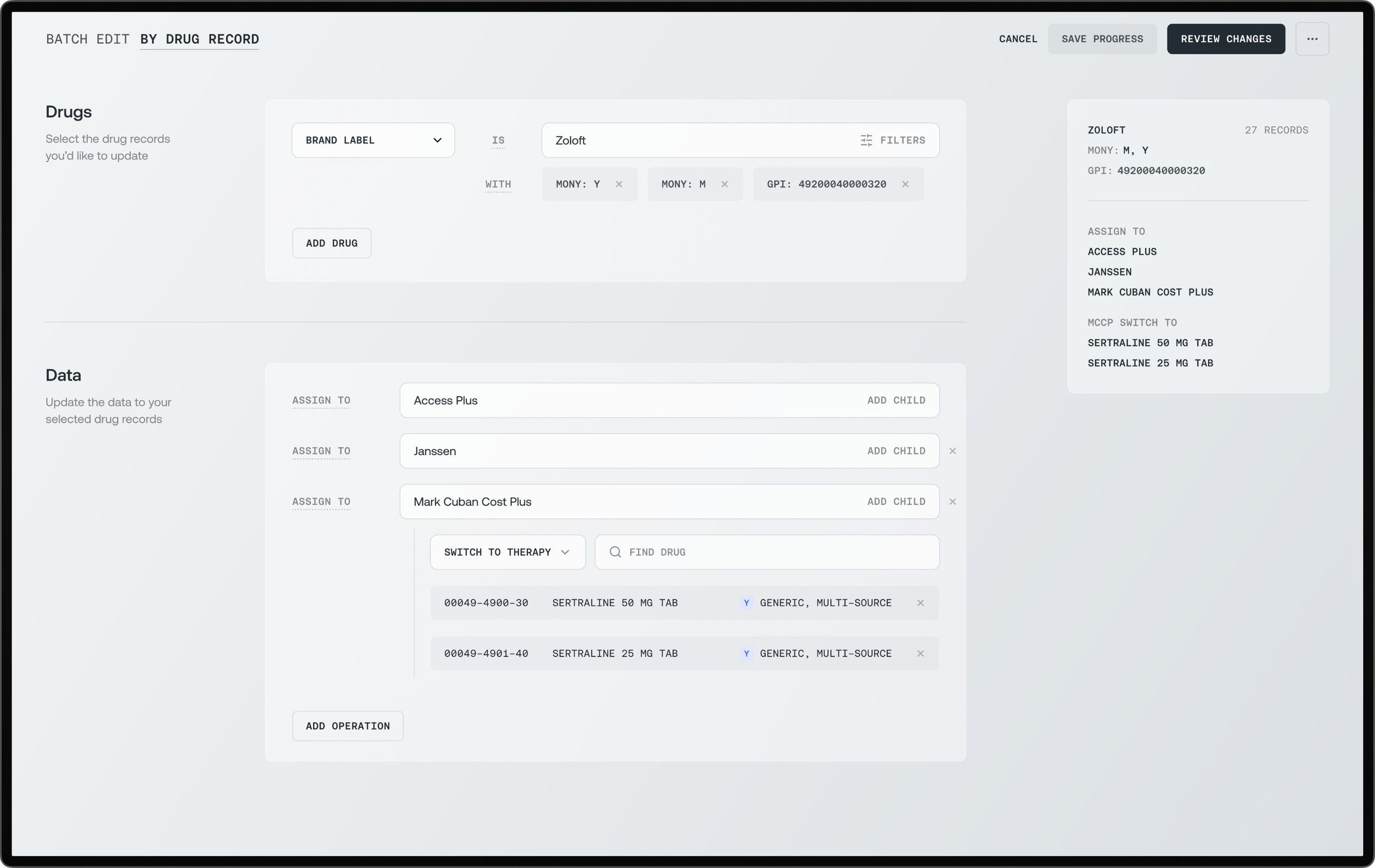
Batch editing operations that adapt to your workflow
I designed a modular system that could work forwards or backwards—matching how different ops specialists think. One user might start with 'assign Zoloft to programs' while another thinks 'update programs by adding Zoloft.' Through extensive workflow mapping and prototype testing, I created interchangeable 'operators' and 'affectors' that made both approaches equally valid. What took 1-2 hours in spreadsheets now completed in minutes—with zero learning curve because the system matched each user's mental model.
Enterprise clarity meets cognitive flexibility—the batch editing interfaces embody Nielsen's heuristic of 'flexibility and efficiency of use' while respecting Miller's Law through progressive disclosure. The monochromatic palette and systematic typography create a 'quiet interface' that reduces cognitive load, while the bidirectional workflow architecture (program→drug or drug→program) honors recognition over recall. Each operation is presented as a discrete, reversible action with a clear visual hierarchy, transforming complex pharmaceutical data management into a series of simple, confident decisions. The design aesthetic deliberately suppresses visual flourish in favor of operational transparency, where the interface disappears into the workflow itself.
Speaking human, not database
Every data point is translated into ops language: structured and identifiable NDC and GPI codes are prominently ordered, with key identifiers being clear and meaningful. Technical fields remained accessible but secondary. Users finally trusted what they saw because it aligned with their expectations.
Drug data humanized through purposeful hierarchy—these drug detail interfaces exemplify the 'Match Between System and Real World' heuristic by translating database schemas into operational vernacular. The design employs Gestalt principles of proximity and similarity to create scannable information clusters: critical identifiers (NDC, GPI) anchor each view, while contextual data cascades in order of operational relevance.
The tri-panel architecture maintains consistency while adapting density to task complexity—embodying Tesler's Law by preserving necessary complexity while eliminating confusion. Alternative therapies appear as actionable cards rather than table rows, honoring Fitts's Law through larger click targets. This is information design that respects both the data's integrity and the human's need for clarity—transforming pharmaceutical complexity into operational confidence through deliberate restraint.
Configurations that anticipate
Smart drug lists and pre-filtered views eliminated the "where do I start" paralysis. Common workflows became one-click operations. The system remembered patterns and suggested next actions based on task context.
Safeguards that prevent disasters
Every high-risk action triggered contextual validation: price changes over 10% required confirmation, decimal anomalies flagged instantly, and bulk updates showed clear previews. The $203.70 pricing error that almost happened? Now impossible.
OUTCOMEUser 'success' testing illuminated the excitement
During user acceptance testing, the excitement became undeniable. Clinical Review crashed our validation sessions just to test their workflows—like Banjo List exports—which transitioned flawlessly because I'd done my homework. Account Managers discovered they could pull AWP pricing and compare it against group rates for custom solutions. Teams scheduled for rollout months down the line wanted in immediately. What began as structured testing became organic advocacy.
Hours → Minutes
Operations drug management tasks
Minimal training
Users were up and running on day one
105% adoption
Exceeded target users
REFLECTIONUnshakeable foundation, transformative at scale
The UAT sessions were so rock-solid that for the first time during my SmithRx tenure, the product launched months ahead of schedule. The early delivery gave me runway to predict, design, and pre-validate the next three significant features: CSV import capabilities, a universal product detail template that could scale across the entire SmithRx ecosystem with dynamic permissions, and programmatic search that would enable true departmental scale. All designed, validated with stakeholders, and planned for rollout by day one launch.
CREDITSLEAD PROGRAM MANAGERPRODUCT MANAGERPRODUCT DESIGN LEADDATA ENGINEERINGConnectOpsCoralyn Beck
Numrin Thaitrong
Aaron Smith
Nathan Xue
Ashton Pigsley